US FDA authorises emergency use of Remdesivir in coronavirus treatment
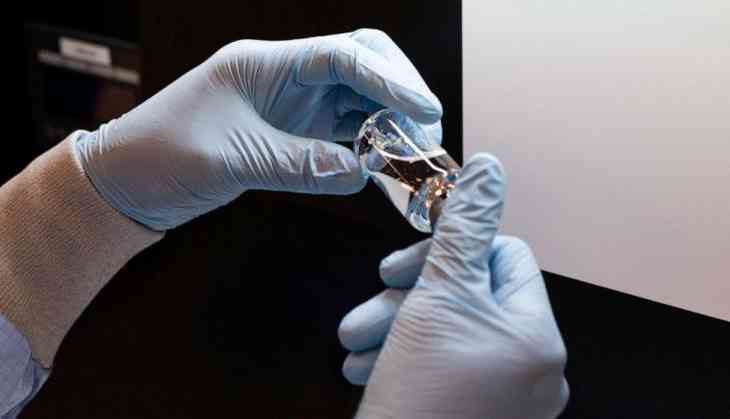
The US Food and Drug Administration has authorized emergency use of Remdesivir drug in coronavirus treatment, FDA head Stephen Hahn announced on Friday.
"We authorized [a pharmaceutical company] Gilead's application for emergency use authorization for the use of Remdesivir in hospitalized patients," Hahn said at a White House event.
US President Donald Trump called Remdesivir to use "a hot thing" and "really, a very promising situation."
Gilead CEO Daniel O'Day announced that the company is donating 1.5 million vials of the medicine.
"We made a decision to donate about 1.5 million vials of Remdesivir. We will be working with the government to determine how best to distribute that. Gilead is fully committed to continuing to expand the supply of the medicine," O'Day said.
O'Day added that Remdesivir clinical trials started immediately when the manufacturer saw "COVID-19 circulating."
(Sputnik/ANI)
Also Read: Coronavirus: UK confirms 1,78,685 cases; death toll at 27,583